Moderna's stock surged after the biotech company partnered with Carsgen to study a new cancer vaccine regimen using mRNA technology, giving Moderna a second opportunity in the field of cancer vaccines.
Antibodies from prior infection or existing vaccines are effective against the BA.2.86 variant, and the increase in COVID cases in the US is not driven by this variant, according to the CDC.
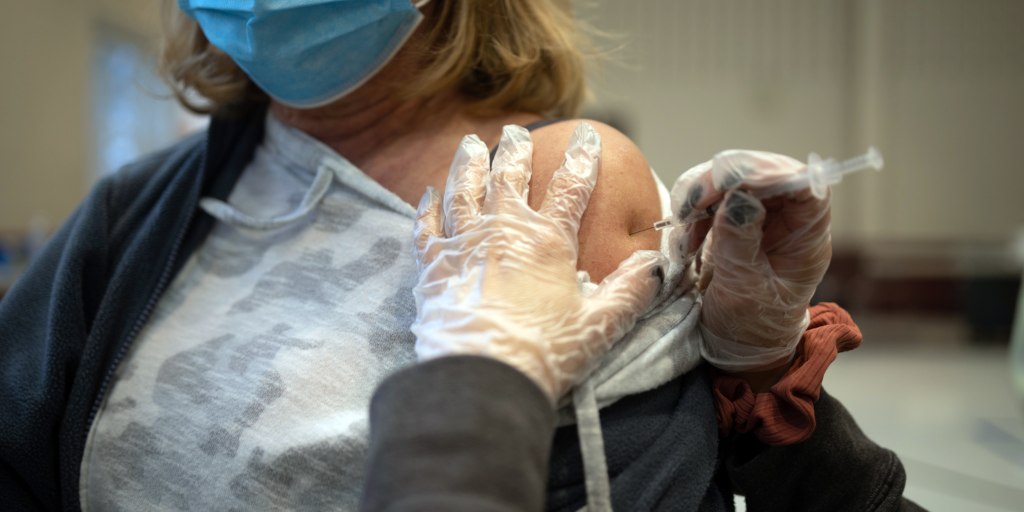
Two new Covid shots from Pfizer-BioNTech and Moderna have been approved by the FDA and are expected to be available soon as cases continue to rise in some parts of the US, with preliminary research suggesting they offer protection against the dominant variant and other concerning variants.
The FDA has approved updated COVID-19 vaccine and booster shots, targeting new variants, and making them available for Americans as young as 6 months old from Moderna and Pfizer.
The US FDA has approved new COVID booster shots from Pfizer and Moderna to address the omicron subvariant XBB.1.5, which could be available in the coming days pending CDC clearance.
Moderna's flu vaccine has demonstrated a stronger immune response against all strains of the influenza virus compared to traditional flu shots, potentially allowing the company to file for accelerated approval in the US by year-end and launch the vaccine for the 2024/2025 season, while also scaling down its COVID-19 vaccine manufacturing to meet lower post-pandemic demand.
Moderna CEO Stéphane Bancel asserts that the positive data on the company's influenza vaccine and their plans to launch 15 products in the next five years demonstrate that they are not solely a Covid vaccine company.